This type of Lewis dot structure is represented by an atomic symbol and a series of dots. From General Chemistry 85 page 314.
Lewis Electron Dot Structures Help
Make sure that H has only 1 bond and that the other atoms do not exceed their normal number 2 bonds to O 3 bonds to N 4 bonds to C and that no atom in.
. O-- needs 4 more dots so they are placed in pairs on either side of the O. Terms in this set 7 Sum the valence electrons from all atoms. Count up all the valence electrons and add them up.
Since Hydrogen is in Group I it has one 1 valence electron in its shell. Determine the total number of valence electrons in the molecule or ion. If ionic treat each ion separately.
Given a chemical formula corresponding to a molecule or molecular ion draw a Lewis structure. Put extra electrons on outer atoms that can accept them. Generally electrons are paired.
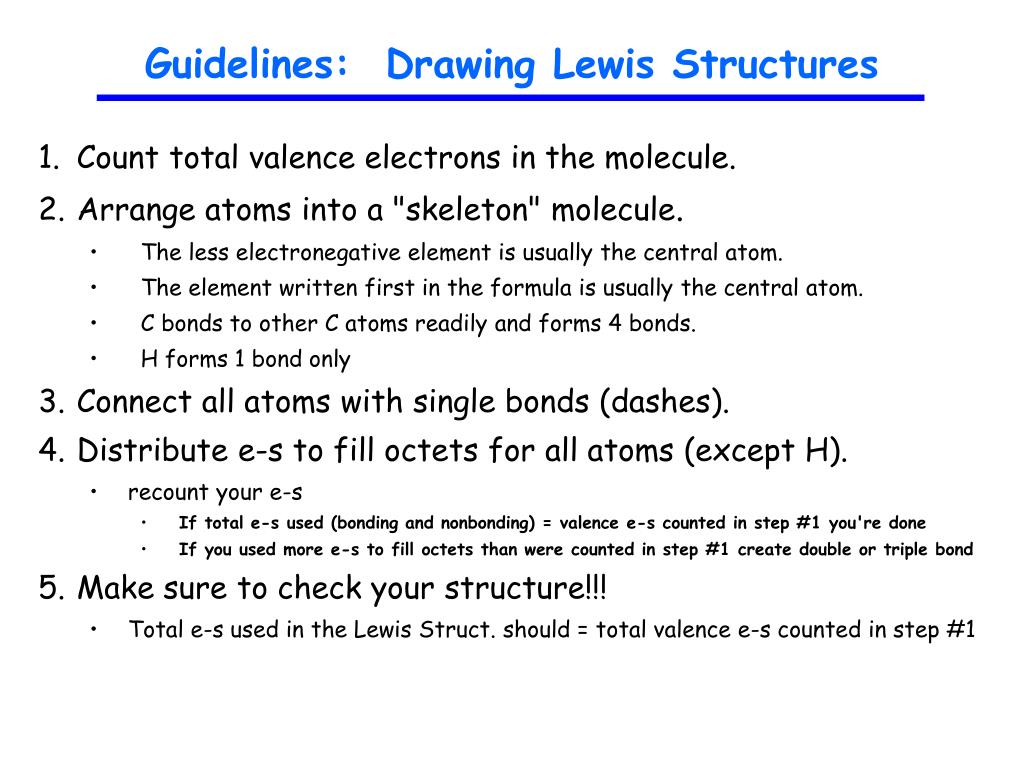
LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1. Rules For Drawing Lewis Structures. Draw the Lewis Dot Structure for the Hydrogen atom.
Decide on a skeletal structure What is bonded to what The central atom is generally written first in the formula Hydrogen is never the central atom even if it is. In drawing Lewis structures the valence electrons are used. For the most part Lewis structures reflect what is found when we gather experimental data for molecules in the laboratory.
APPENDICES 1 GUIDE Lewis Structures formal charge and oxidation numbers. All valence electrons of the atoms in Lewis structures must be shown. Rules for Drawing Lewis Structures Electronegativity - a measure of the tendency of an atom to attract a bonding pair of electrons.
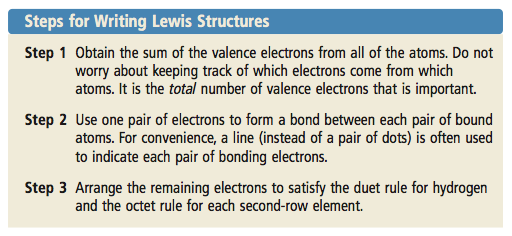
1 Find the total number of valence electrons for a neutral molecule by adding up the number of valence electrons needed for each atom in the molecule. -Subtract one electron for each positive charge. The general rules for writing Lewis structures include the following.
If covalent treat the entire molecule. Lewis electron dot Structures. View Drawing_Lewis_Structurespdf from SLE 133 at Deakin University.
A video tutorial for how to draw Lewis Structures in five steps. Place the dots in pairs. The Octet Rule actually applies only.
Join learners like you already enrolled. One way to do this is to write the Lewis symbols for all of the atoms in the formula and count up all the dots. Determine whether the compound is covalent or ionic.
If the charge is negative add electrons and if the charge is positive subtract electrons. Rules for drawing Lewis Structures. Once the electron dot structure is complete translate this structure into a Lewis structure.
The electrons pair between two atoms are the bonding pairs and electrons that are not in between two atoms are the nonbonding electrons lone pairs. For example two isomeric Lewis structures can be written for C 2 H 6 O and four can be written for HCNO. There are two rules to follow when placing the remaining dots.
Copyright 2001 L. Arrange atoms in a bonding configuration. -Hydrogen cannot be central.
Write the skeletal structure. Least electronegative atom typically goes in the middle. H gets arrayed around the outside.
General rules for writing LEWIS structures for molecules. A more userfriendly approach Note 1 JOHN E. B Make sure all other elements have 8 dots surrounding them.
Rules for Drawing Lewis Structures Complete the Lewis structures in the table on the datasheet. Compounds of low electronegativity metals with high electronegativity nonmetals ΔEN 16 are ionic as are compounds of metals with polyatomic anions. Rules for Drawing Lewis Structure.
First of all a correct count of all valence electrons is essential. Note the charges on anions and. Unpaired electrons are observed in odd electron molecules such as NO and NO2.
To do this you will need the periodic table. For a molecule simply sum up the valence electrons of the atoms present. Two electrons are shared between two atoms constitutes a single bond.
Lewis structures can be drawn with a set of general rules that give us a pretty good idea of how valence electrons are distributed around a molecule. Ad Find the right instructor for you. MUST FOLLOW IN ORDER.
The following rules are given to assist you. For a polyatomic anion electrons are added to take into account the negative charge. Rules for Drawing Lewis Structures For Molecular Compounds 1 Calculate the total number of electrons for the structure by summing the valence electrons of each atom in the molecule.
Count the number of valence electrons. Using the periodic table use the group number to determine the number of valence e-. Up to 24 cash back Draw a skeleton structure for the molecule or ion by joining atoms by a single bond to the central atom.
See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. The video covers the basic Lewis structures youll see in an introductory chemistry class. Draw the initial skeletal structure with atoms connected by single bonds.
For a molecule uncharged that count is the correct. -Add one electron for each negative charge. A Make sure each H atom only as two dots surrounding it.
Steps of drawing lewis structure of BH 3. It should be the most symmetric structure you can draw. Lewis is a structure of drawing that reveals both bonding and nonbonding electrons pairs.
Put the electron in- 2 electrons for each single bond between atoms. Determine the total number of valence electrons in the molecule. Count the total number of valence electrons in the structure Remember Group of Valence electrons 2.
Choose from many topics skill levels and languages. Rules for Drawing Lewis Structures For molecules known to exist follow these rules to determine the Lewis structure. Its not perfect but it is a useful tool for scientists trying to understand how molecules will react and their.
Four electrons shared is a double bond and six electrons shared is a triple bond. Although four of the five cases in Rule 6 require no alteration of the structure each case represents a normal bonding condition for the atoms involved. -Atoms that occur once or first are usually central.
And recall that for main group elements the valence electrons can be determined from the group number. Rules for drawing Lewis structures. CBr 4-The least electronegative atom is central.
In the example refer to the picture H -- already has 2 dots. Lewis bases are also Brønsted bases.
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949

Electron Dot Model Of Bonding Lewis Structures

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421

I Can 2 I Can Draw A Lewis Structure Rules For Lewis Structures 1 Total Number Of Valance Electrons 2 Central Atom Always C Never H Rarely O Or Ppt Download



0 comments
Post a Comment